When we think of light, we think of the light that we can see with our eyes – sunlight, light from electric lights, light from a fire or candle etc. But there is a lot of light that our eyes cannot see, for example infrared or ultraviolet.
A more accurate phrase for light energy is ‘electromagnetic radiation’ which means exactly the same thing scientifically; Electromagnetic radiation includes all the light we can see, and all the light we can’t.
It can seem a bit strange to describe light we can’t see as ‘light’, since it appears dark to our eyes. But scientifically speaking, all light – all electromagnetic radiation – is the same thing whether we can see it or not. Some creatures can see colours of light that we can’t– for example, there is a snake called the pit viper that can see infrared, a colour that appears completely black to our eyes1.
Table of Contents
Examples of Light Energy
When talking about different colours of light energy, scientists use the terms ‘wavelength’ or ‘frequency’ to describe it. Where we might say, “the colour of that light is red” a scientist might say “the wavelength of that light is 700 nanometres” or “the frequency of that light is 400 terahertz” – these three statements all mean the same thing. For the rest of this article, I shall refer to the ‘colour’ of electromagnetic radiation by its wavelength.
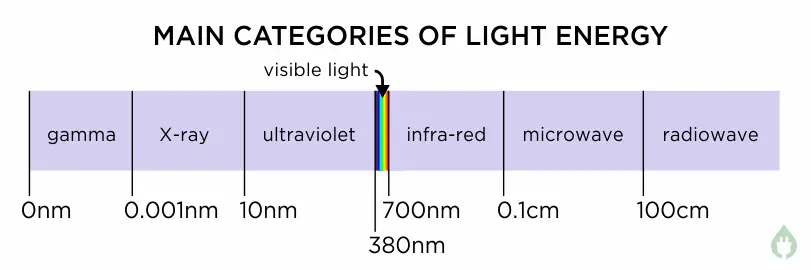
Let’s take a look the whole range of all light energy – this is called the electromagnetic spectrum. We can divide it up into the seven main categories to look at the most common examples.
1. Visible Light
This is the best place to start in the electromagnetic spectrum – light that we humans can see. For convenience, visible light wavelengths are usually measured in nanometres (written nm – one nm is a millionth of a millimetre). Visible light starts at a wavelength of about 700 nm (a deep red colour) and goes down to about 380 nm (a deep violet colour)2. This means visible light occupies a range of wavelengths about 320nm wide. As can be seen on the chart above, this is actually a very tiny range of colours compared to the whole electromagnetic spectrum.
Most of us have seen a rainbow in the sky. These occur when light energy from the sun is split up (or ‘refracted’ as scientists call it) into all the different wavelengths of visible light – red, orange, yellow, green, blue, indigo and violet – and we see them as bands of colour in the sky. Here is a chart showing all the colours and their approximate wavelengths:3
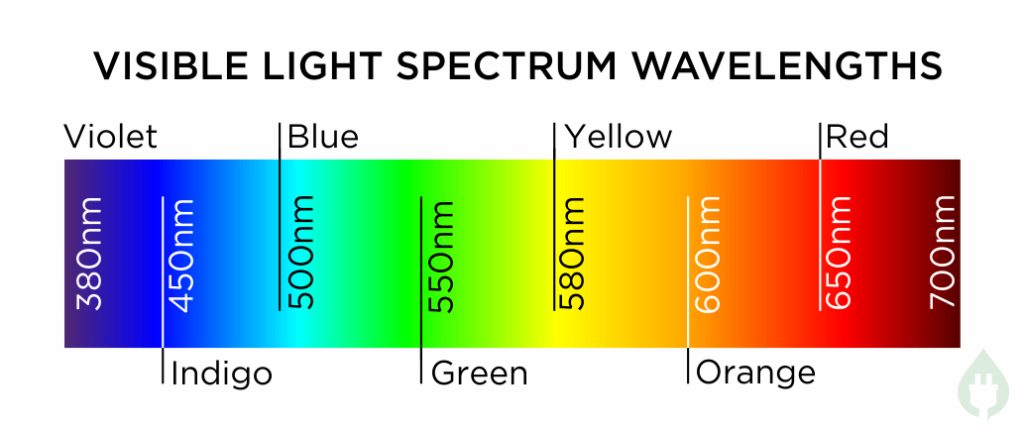
Note this chart is shown in reverse colour order to normal – this is to show the increasing wavelength of the light going from 380nm at the left (violet) to 700nm at the right (red).
You might wonder why white light isn’t shown in the spectrum of visible light colours. This is because white light is made up of an equal mix of all colours (wavelengths) of light. If you look with a magnifying glass at the pixels of a television screen or computer monitor, you will see that any white areas are actually made up of separate red, green and blue pixels. These three colours mix together in our eyes and we see them not as three individual colours, but as white.
Another thing to note about visible light is that at the violet end where the wavelength drops down below about 380nm, and at the red end where the wavelength goes up above about 700nm, red and violet colours get darker and darker. This is because our eyes cannot see light at wavelengths below 380nm or above 700nm, and so they seem black to us, even though there is still light there.
2. Infra-red Light
Just outside the colour range of our eyes, light that exists off the right of the red end of the visible spectrum is called infra-red light. Infra-red light wavelengths start just beyond red, at about 700nm, and go all the way up to about 1,000,000nm4. This range of wavelengths is over 3000 times wider than the range of visible light.
Infra-red light energy is also sometimes called thermal radiation, since it is emitted by anything that gives off heat. For example, the sun gives off huge amounts of infra-red light energy, as does fire.
All warm-blooded animals (humans included!) give off small amounts of infra-red light energy. This light can be seen by special infra-red cameras, even in pitch black. Infra-red cameras can also be used to remotely measure temperature, since the wavelength and intensity of the infra-red light given off by an object/person/animal is directly proportional to its temperature.
3. Ultra-violet Light
Going off the left hand end of the visible spectrum wavelengths, we find ultra-violet light. Ultra-violet begins just to the left of 380nm, and goes all the way down to about 10nm.5
Within the ultra-violet range, there are four ‘colours’ – although using the word ‘colour’ is a bit misleading for light energy that is invisible to our eyes, so I shall call them ‘wavelength bands’.
The four ultra-violet wavelength bands are:
| 380 – 315nm UV-A | Also known as ‘blacklight’, UV-A is used in special lamps that make white objects seem to glow. Most of the light given off by a tanning sunbed is in the UV-A band. UV-A is also given off by the sun. |
| 315 – 280nm UV-B | A small amount of the light from a tanning sunbed is in the UV-B range. UV-B is also given off by the sun. UV-B has been shown to cause melanomas (skin cancers) after excessive exposure. |
| 280 – 100nm UV-C | Given off by the sun but none of it reaches us at ground level as it is scattered back into space by the earth’s atmosphere. |
| 100 – 10nm Extreme-UV | 10nm Extreme-UV ; lasers that emit extreme-UV light energy are used to make modern GPU and CPU silicon chips that go inside graphics cards and computers.6 |
4. X-rays
After passing down through extreme-UV, the next wavelength band along is the X-ray band. This goes from 10nm down to 0.001nm7. X-rays were accidentally discovered in 1895 by Wilhelm Roentgen, a physics professor in Bavaria8.
X-rays are now commonly used across the world in medical diagnostic imaging, due to their ability to shine through human flesh and allow inner solid objects like bone or cancer masses to be visible on a photograph.
X-rays are a high energy form of electromagnetic radiation, and as such are sometimes referred to as “ionizing radiation”, which means they are capable of damaging human tissue. Long-term, repeated exposure of human tissue to X-rays has been shown to cause cancer.
5. Gamma Rays
Once we go past X-rays, we pass into the realm of very high energy radiation, and this wavelength band is called the gamma ray band. X and gamma rays meet at around 0.001nm, but in the same way that you could describe 590nm wavelength light both as “yellowy orange” or “orangey yellow”, there is some overlap at the boundary where X rays and gamma rays meet. One scientist might describe a wavelength of 0.001nm as a small wavelength X-ray (yellowy orange), whereas another scientist might describe it as a long wavelength gamma ray (orangey yellow).
There is no theoretical limit to how small a gamma ray wavelength can go. The smaller the wavelength, the higher the energy in the radiation, and the more dangerous it is.
The term gamma ray was first used by British scientist Ernest Rutherford in 1903, to describe the radiation given off by radioactive elements like uranium, thorium and actinium.
Gamma rays are also a form of ionizing radiation, and as such are exceedingly dangerous, even more so than X-rays. Whilst uncontrolled gamma radiation exposure can cause cancers, highly targeted and focused gamma rays are used in medical therapies to attack and kill cancer cells in what is called radiation therapy9.
6. Microwaves
We are now moving away from the very short wavelengths, back up through visible light and infra-red light. At the opposite end of infra-red light (about 1,000,000nm) we enter the realm of microwave radiation.
At these longer wavelengths, for convenience, scientists use centimetres (cm) rather than nanometres. Microwaves span the wavelength range 0.1cm (1,000,000nm) up to about 100cm.
Microwaves are generated using a piece of equipment called a magnetron, and while they were originally used for radio transmissions (microwaves are very short wavelength radio signals), they are now best known for their use in cooking. This is because moisture absorbs microwave radiation, heating up as it does so, which is why microwave ovens will not heat any object that has no moisture in it.
In a very curious twist, it was a British scientist, James Lovelock, who inadvertently invented the microwave oven, when he used a Faraday cage attached to a magnetron emitter to bake a potato as he was working on different methods to warm up frozen hamsters in a 1950s biology experiment.10
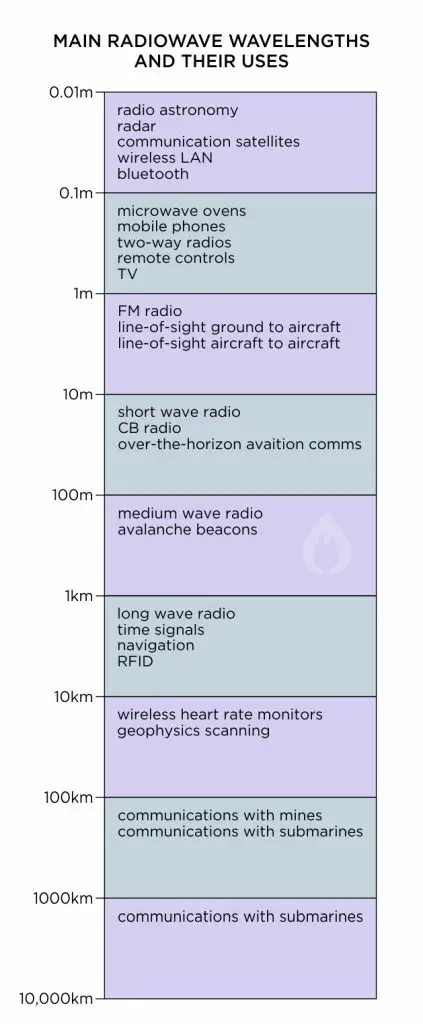
7. Radio Waves
Above 100cm, at the far right hand end of the wavelength spectrum, we come to radio waves. As mentioned above, microwaves are also a form of radio waves, but they are usually separated out from other radio waves due to their ability to be absorbed by moisture, which longer-wavelength radio waves cannot.
Radio waves are used for all forms of wireless communications. From Bluetooth to Wi-Fi, CB radio to mobile phones (cell phones), these all use radio waves. The radio wave bands are further split up into separate sections depending on what kind of communications they are used for.